MEDALLOY Doctoral Network
APPLICATIONS NOW OPEN!
Please see Applications for the link to the application portal and instructions.
Medalloy Doctoral Network
Recruiting 9 Researchers
MEDALLOY is a Marie Skłodowska-Curie Actions (MSCA) Industrial Doctoral Network which will develop advanced Nitinol alloys for medical device applications. MEDALLOY will provide world-class training for nine doctoral candidates (DCs) through an international consortium across six countries, which consists of four academic, two clinical and five industry members who together operate across the entire Medical Device Value Chain, including material production, manufacturing and delivering devices. MEDALLOY researchers will enable the next generation of Nitinol devices by optimising manufacturing processes, enhancing defect tolerance and predicting fatigue life and device performance.
Our Objective
Nitinol is an advanced alloy that is widely used and of critical importance in modern medical devices such as stents, heart valves, and guidewires due to its excellent biocompatibility and unique superelasticity and shape memory behaviour. The deployment of Nitinol in these and other devices has revolutionized treatments for widespread conditions such as atherosclerosis and valvular heart disease, directly addressing Europe’s growing burden of cardiovascular disease.
Our research objectives are:
Our training objectives are:
Our Consortium
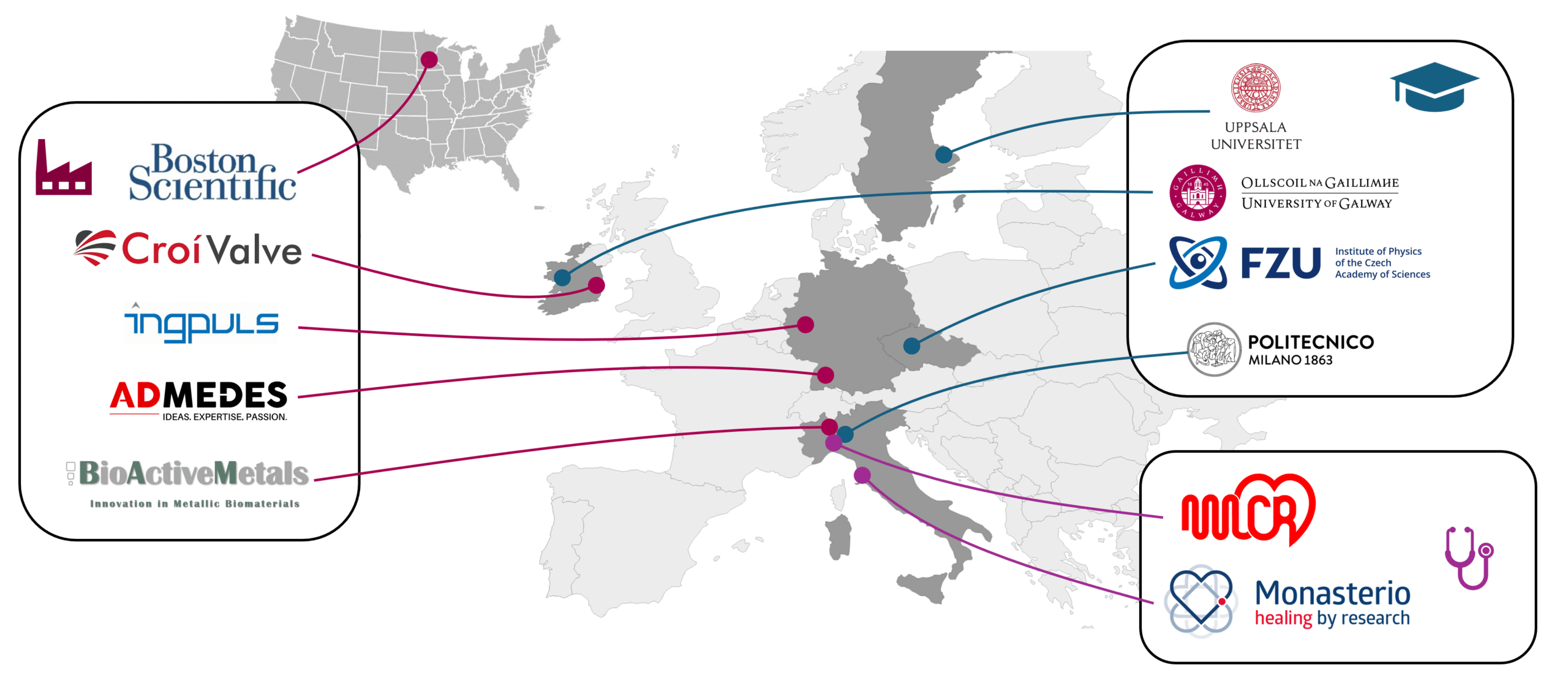
The MEDALLOY consortium brings together academic, industrial, and clinical organisations with established expertise in Nitinol materials, medical device development, and biomechanics. It includes three degree-awarding institutions (University of Galway, Politecnico di Milano, and the Institute of Physics of the Czech Academy of Sciences) and our industrial partners Admedes, BioActiveMetals, Boston Scientific, CroiValve, and Ingpuls are involved across the Nitinol medical device supply chain from alloy production to finished devices. Our network is supported by partners who provide clinical, regulatory, ethical, and commercialisation expertise to support the research and training actions. The network is structured to support the MSCA Doctoral Network requirements, with joint supervision across sectors, formal progress-monitoring procedures, and coordinated training activities. Partners contribute laboratory infrastructure, industrial-scale manufacturing capabilities, simulation tools, and clinical facilities relevant to the individual doctoral projects, and participate in shared management, technical committees, and review processes.

Coordinator
The Medalloy Doctoral Network is Coordinated by Dr William Ronan from the Institute for Health Discovery & Innovation and the School of Engineering at the University of Galway, Ireland.
The Institute for Health Discovery and Innovation at the University of Galway is an interdisciplinary, cross college, institute focused on supporting high quality research activity across the domains of health discovery and innovation research.
Dr Ronan is the Vice Dean for Graduate Studies at the College of Science and Engineering at the University of Galway and previously the training coordinator for the MSCA Innovative Training Network BioImplant
Dr Ronan’s research group focuses on materials for medical devices including superelastic Nickel-Titanium alloys, biodegradable polymers and other advanced materials. His group uses computational solid mechanics and multi-physics modelling to develop and understand the next generation of materials for medical devices.

This project has received funding from the European Union’s Horizon Europe research and innovation programme under the Marie Skłodowska-Curie Actions grant agreement No 101227596
Funded by the European Union. Views and opinions expressed are however those of the
author(s) only and do not necessarily reflect those of the European Union or the European
Research Executive Agency (REA). Neither the European Union nor the granting authority
can be held responsible for them.